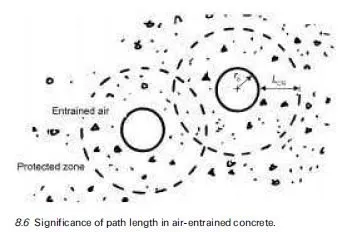
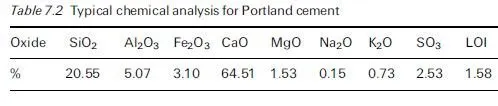
For concrete in contact with aggressive ground, it is obviously desirable that cement with a good record of sulfate resistance is used, e.g. a suitable extended cement containing ground granulated blastfurnace slag (ggbs) or pulverised fuel ash (pfa) which has been adequately cured prior to exposure. The concrete should be of low penetrability, having an appropriately low water/cement ratio, achieved through the use of admixtures where necessary, to minimise the rate of ingress of extraneous sulfates from the environment into the material. Detailed guidance applicable to UK exposure conditions has been given in BRE Special Digest 1, which has already undergone significant revisions since it was first introduced in 2001 and is currently in its 3rd edition (Building Research Establishment, 2005), as discussed in Section 4.5. One of the many difficulties in formulating simple guidance rules stems from the fact that the conditions to which concrete is exposed prior to coming in contact with sulfate-bearing groundwater can have a marked influence on the severity of sulfate attack. Thus even mild atmospheric carbonation can play a significant role in modifying the surface pore structure of the material, thereby reducing its penetrability. Current guidance recognises this effect in providing protection to precast products which are exposed to non-acidic sulfate-bearing environments (Building Research Establishment, 2005).
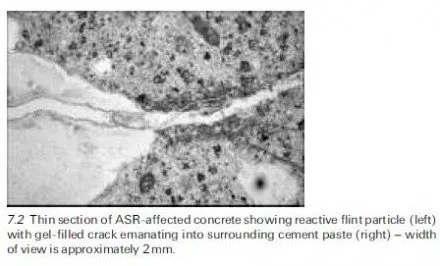
As already mentioned, a recent review has highlighted several other features that contribute to the confused state of knowledge of sulfate attack (Neville, 2004). Amongst these is the influence of the cation, with sodium, calcium and magnesium sulfates all having different effects. Sulfate-resisting Portland cement (ASTM Type V cement), which was developed to minimise reaction with hydration products of the C3A (aluminate) phase does not offer special protection against attack upon C-S-H or CH and the combined presence of Mg2+ and SO4 2ÿ ions can result in the conversion of these hydrates to magnesium hydroxide (brucite), silica gel and gypsum. The concomitant lowering of the pore solution pH, due to the low solubility product of brucite (for which a saturated solution has an equilibrium pH of ~10.5), destabilises the C-S-H and accounts for the fact that magnesium sulfate is more aggressive than sodium sulfate or calcium sulfate. If the pH is maintained at an artificially high level, however, as in some laboratory tests involving magnesium sulfate, unrealistic results may be obtained because brucite can sometimes form an insoluble protective layer upon concrete unless it becomes mechanically damaged. Altogether the state of current knowledge of external sulfate attack under field conditions remains inadequate and although the problem is generally avoidable by following prescriptive guidance of the kind given in BRE Digest 1 (Building Research Establishment, 2005) it is difficult to devise appropriate laboratory tests for assessing the resistance of different types of concrete subject to different exposures; this problem with performance tests is discussed in further detail in chapter 9 of the book by Skalny et al. (2002). Various attempts to devise mathematical models that might serve as aids in predicting per- formance have been made by researchers in this field and a recent review of the work appears in chapter 7 of the book by Skalny et al. (2002) ± see also Section 4.6.4 of the present chapter. Unsurprisingly, however, it is fair to say that progress to date has been rather limited.