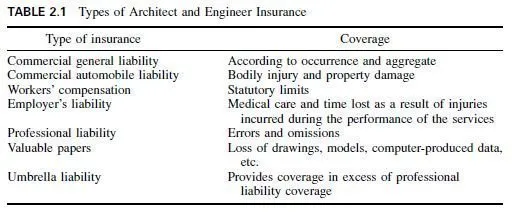
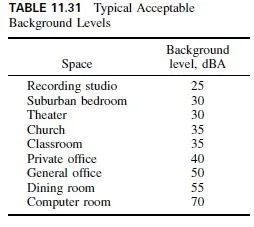
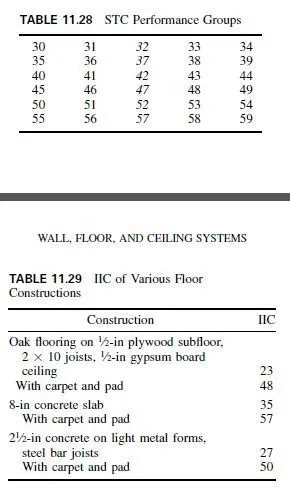
Originally, the bronzes were all alloys of copper and tin. Today, the term bronze is generally applied to engineering metals having high mechanical properties and the term brass to other metals. The commercial wrought bronzes do not usually contain more than 10% tin because the metal becomes extremely hard and brittle. When phosphorus is added as a deoxidizer, to obtain sound, dense castings, the alloys are known as phosphor bronzes. The two most commonly used tin bronzes contain 5 or 8% tin. Both have excellent cold-working properties.
Silicon Bronze
These are high-copper alloys containing percentages of silicon ranging from about 1% to slightly more than 3%. In addition, they generally contain one or more of the four elements, tin, manganese, zinc, and iron. A typical one is high-silicon bronze, type A.
High-silicon bronze, A (96.0% copper, 3.0% silicon, 1.0% manganese). Typical users are tanks pressure vessels, vats; weatherstrips, forgings. General properties are corrosion resistance of copper and mechanical properties of mild steel.
Aluminum Bronze
Like aluminum, these bronzes form an aluminum oxide skin on the surface, which materially improves resistance to corrosion, particularly under acid conditions.
Since the color of the 5% aluminum bronze is similar to that of 18-carat gold, it is used for costume jewelry and other decorative purposes. Aluminum-silicon bronzes are used in applications requiring high tensile properties in combination with good corrosion resistance in such parts as valves, stems, air pumps, condenser bolts, and similar applications. Their wear-resisting properties are good; consequently, they are used in slide liners and bushings.