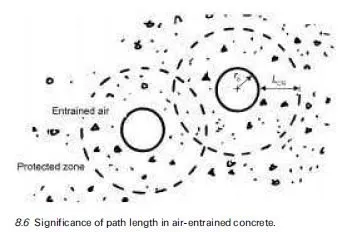
The relationship between volume and temperature in a given state is significant in materials with a high coefficient of thermal expansion. The volume change during a change of state is of even greater interest in the case of water in concrete. The change of water density in a temperature range from ‡30ëC to ÿ30ëC leads to a situation whereby water on freezing to ice has a volume approximately nine per cent greater than that from which it was formed (Neville, 1995).
The freezing process begins within the capillary pore structure. The volume increase of the pore water is of immediate concern in saturated concrete but freeze-thaw damage is exacerbated by additional factors. One such factor is water supply to growing ice crystals induced by osmotic pressure. This is related to concentration gradients caused by redistribution of solutes (Powers, 1975).